- Online only



Let us notify you about our product updates.
Mynox® is a mycoplasma removal agent for treatment of contaminated cell cultures. Mycoplasma elimination is achieved in only 6 - 8 days (plus 4 passages for DNA washout).
The classic Mynox® avoids the use of typical antibiotics and instead contains surfactin, a cyclic lipopeptide. This prevents the development of resistant strains. The mode of action of Mynox® is based on a biophysical mechanism to integrate into the membrane of mycoplasmas and thereby impair their integrity
Mynox® is intended for research use only. Not applicable for clinical treatment. Protocols for the direct treatment of viruses are described in the product manual.
Mynox® is a sterile, ready-to-use solution, aliquoted per tube for single use, 200 µl per vial.
1 application can be used to permanently eliminate mycoplasma from one cell culture.
Successful elimination of mycoplasma should be verified with a suitable mycoplasma detection system, e.g. Minerva Biolabs’s Venor®GeM Mycoplasma PCR Detection Kits.
Tissue culture petri dish (60 mm Ø)
Standard cell culture plasticware
Standard cell culture equipment (incubator, centrifuge, pipettes, etc.)
Store the components at +2 °C to +8 °C until the expiry date indicated on the label.
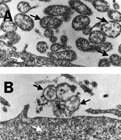
Fig. Electron micrograph showing the effects of Mynox® when applied to contaminated cell cultures. (A) Mycoplasma contaminated cell culture before treatment. (B) With one application of Mynox® membranes of all mycoplasma are destroyed while the tissue cell membrane is unaffected. Black arrows indicate mycoplasmas, white arrows indicate parts of a tissue cell.
Minerva Biolabs offers a free sample of this product for testing:
Request free sample